Eric Raabe MD, PhD
One of the goals of pediatric cancer treatment is deploying personalized medicine. Treating each patient and each tumor, with exactly the right type of therapy to maximize killing of tumor cells and minimize side effects to patients is the objective. Initially, brain tumors were classified by their appearance under the microscope. Medulloblastoma, for example has a characteristic appearance that is different from ependymoma.
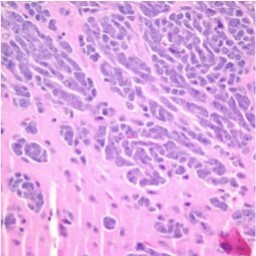
Researchers discovered that certain tumors expressed fairly specific tumor markers. For example, medulloblastoma can be differentiated into subtypes, based on expression of some key proteins. Beta-catenin is expressed in some tumors while others express a gene called Hedgehog. Patients that have beta-catenin positive tumors have a better prognosis than High power view of a model of high risk medulloblastoma, showing tumor cells (blue) invading into the surrounding normal mouse brain (pink). those with other types of medulloblastoma. This allows for “risk stratifica-tion,” meaning that patients with beta-catenin expressing tumors might not need as much therapy as those with some other types of medulloblastoma. The next level of progress was RNA profiling which shows the expression of thousands of genes simultaneously. Several of these large-scale studies in medulloblastoma have shown that medulloblastoma consists of four different tumor types, each with a different prognosis.
Unfortunately, this type of profiling is very expensive and isn’t applicable for routine use. Researchers are trying to come up with a few genes that will function as genetic fingerprints that they can use to subdivide medulloblastoma and help clinicians decide what therapy to use in each patient. Different specifically targeted therapies are being developed to kill medulloblastoma cells from each of the subtypes. Knowing which one will help researchers discover the correct therapy, designed for a specific tumor. While this therapy isn’t being utilized right now, we are waiting for the development of diagnostic tests to tailor brain tumor therapy for each patient. The goal is to develop therapeutics that will target the “Achilles’ heel” of each tumor subtype.
We are seeing some of these drugs now in early clinical trials in medulloblastoma and other brain tumors. It is most likely that targeted therapy will be used in association with traditional therapies such as radiation and chemotherapy. As more finely tuned drugs are developed, it may be possible to improve cure rates while decreasing a child’s exposure to the side effects of radiation, surgery, and chemotherapy. The promise of this type of targeted therapy is that future patients will receive the correct ther-apy while minimizing side effects.
Eric Raabe MD, PhD, Instructor of Pediatric Oncology
The Johns Hopkins Pediatric Neuro-oncology Service
Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital
